Metal plating has for centuries been a popular method to provide objects or machine parts with additional properties of specific metals for desired results. Plating could be used for a wide variety of applications. People use it to give objects a metallic look for aesthetic reasons. They also use it to alter a component’s physical properties for better performance.
Metals or metal alloys like gold, silver, copper-tin, aluminium, cadmium, zinc, chromium, or nickel are used in the plating process. The choice depends on the application goal. In the case of mechanical components, metal plating is done to improve their corrosion resistance, reduce friction, and increase their durability and strength.
Alloys that combine two different metals can bring added advantages. They also help reduce costs without compromising quality. For instance, using a zinc-nickel alloy confers the best of both metals on the plated component. The alloy improves corrosion resistance significantly. It prevents the formation of white rust for up to 500 hours and keeps red rust at bay for up to 1000 hours during salt-spraying testing.
Types of metal plating process
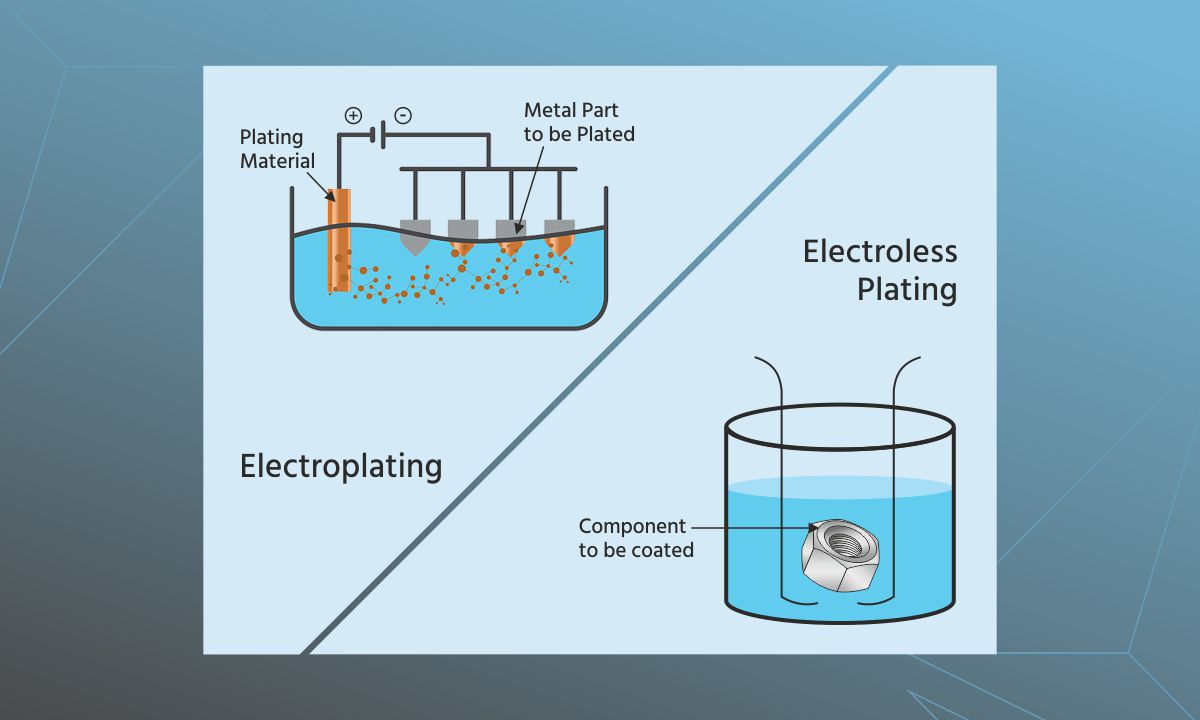
The metal plating process is of two different types: Electroplating and electroless plating. Electroplating requires an electrical current. Electroless plating involves dipping the component in a liquid solution to bring about the desired reaction. Both the processes have their own pros and cons. The choice of plating method is determined by the application type of the component and costs.
Electroplating method
This process uses electrical current to achieve uniform metal plating. The current reduces dissolved metal cations that are nothing but positively charged ions. Electrodes are the key components of the electroplating process. The material to be plated is also a cathode. It is negatively charged. The metal to be coated on the object is the positively charged anode. Both these components are immersed into an electrolyte solution comprised of metal salts and other ions to facilitate the proper circulation of electrical current. The current then leads metal cations to form a thin coating onto the object to be plated. This process is called electrodeposition.
In electroplating, an external power source must supply electrical current to the anode. This process oxidises and dissolves metal atoms into the electrolyte solution. These dissolved ions then go on to plate the object. The ions present in the electrolyte are replenished throughout the process by the anode which is made up of the metal used for plating the object. At the end of the process, the chemical properties of the component. are altered. Its resistance to corrosion improves, dulling is prevented, increasing the component’s durability, and it gains added tensile strength. A part that could not perform under pressure might be able to do so after plating.
Electroplating is the best method to achieve a uniform thickness of the coating. Despite a high consumption of power, it is an economical method due to the high volume of components to be plated. This process has other benefits, such as uniform surface abrasion and building the metal substrate’s thickness. Electroplating enhances the surface of components, making them ideal for plaining. Check out the advantages of electroplating as below.
The advantages of electroplating include as follows:
-
It enhances the visual appeal of objects, giving them a shiny, polished finish.
-
Electroplating can improve the durability of an object by adding a layer of protection against wear and corrosion.
-
It provides an added layer of protection, making objects more resistant to scratches and wear.
-
In the food industry, electroplating with tin is used to safeguard canned food from spoilage.
-
Electroplating economical metals with silver or gold helps lower the production costs of ornaments and items.
-
Electroplating can improve an object’s conductivity. This makes it more suitable for electrical applications.
Sargam electroplating advantages offers a key benefit of strengthens materials against rust and corrosion. Through this process, a protective metal layer forms on the surface of the substrate, safeguarding it from environmental damage and prolonging its durability.
Electroless method
The electroless method uses an auto-catalytic chemical reaction and not an electrical current to plate components. This process involves dipping the component in a liquid to induce multiple reactions at once. Electroless plating does not need two electrodes and an external power source. It uses one electrode and relies purely on chemical methods. This also makes electroless plating less costly.
Electroless plating uses a reducing agent for plating. Nickel tends to be the most commonly used metal in this process. However, other metals such as copper, silver and gold can also be plated onto components using electroless plating. This method does not need electrolyte baths or external application of current. It allows more flexibility in the shape and size of objects to be plated and can deliver varying levels of finishing brightness in the coating. On the downside, however, electroless plating tends to be much slower and cannot deliver the desired thickness of the coating that electroplating can. It is still a very effective method to alter the physical and chemical properties of components with effective coating. It also improves their durability.
Selective plating or brush plating
Selective plating or brush plating is a method related to electroplating. It is used when plating is needed in more localized areas of components.
The brush is a piece of stainless steel metal wrapped in cloth. It holds the plating solution and prevents the component from being plated from making direct contact. An operator does the localized plating with the brush using a low voltage current. Achieving uniform localized plating with a brush requires considerable skill. Brush plating enables specific spot plating. It’s mostly useful for repairing and refurbishing components. Electroplating involves giving a full component an electrolyte bath. In contrast, brush plating applies the electrolyte plating solution to a targeted area and uses an anode connected to a wire.
Brush plating uses a brush that is flexible and easy to maneuver brush attached to a power supply. This makes the process appear akin to welding in comparison with other electroplating methods. The slight difference between brush plating and full-fledge electroplating is that in brush plating, the anode is connected to a handle and wrapped in a cloth. The object to be plated is still the cathode. Moving anode over the cathode completes the circuit and enables electrolytes to be supplied continuously. An electrolyte is generally supplied by a pump or by dipping. The cloth works to absorb electrolytes in the brush plating process.
Advantages of brush plating
Brush plating should be considered whenever metal surface enhance build up for re-sizing or mechanical repair is required. Brush plating has numerous advantages over other repair methods such as, thermal spray, welding or tank plating. The brush plating process is widely used across a range of industries. It includes aerospace, oil and gas, marine, petrochemical, and more.
Mobility and flexibility
Brush plating is much more flexible than electrolyte baths as the equipment for localized plating is mobile. Brush plating can be done in any location ranging from onsite to customer location or your own workshop. It does not involve the risk of transporting heavy and delicate machinery for plating.
Speed
Brush plating reduces the need for machining as it enables the deposition of metal onto a component in thin layers. There are no limits to the thickness of plating that can be achieved by this method. Due to its compact equipment and precise procedure, brush plating is much faster than conventional electrolyte baths.
Precision
Selective plating allows plating of small spaces which need the very low volume of metal. These characteristics make it ideal for the repair and refurbishment of components.
Other perks
-
The need for masking is reduced in brush plating
-
Can service large parts which cannot be addressed by electrolyte baths
-
Needs less chemical volume
-
Consumes less electrical power and reduces power expanses
-
Has more speed and can be performed on-site.
Limitations of brush plating
Less economical
Selective plating or brush plating is not as economical as traditional electroplating or electroless plating. Despite its compact size and accuracy, selective plating or brush plating is not as economical as traditional electroplating or electroless plating. This difference arises due to the volume of material involved in traditional plating methods.
Need for skill
Unlike traditional electrolyte baths, selective plating is a more hands-on process that needs a skilled operator trained in brush plating.
Applications of selective or brush plating
Selective plating is used in a number of sectors from manufacturing to energy and aerospace. It is useful for repairing, refurbishment of components, and reduces downtime considerably by keeping critical machines functioning.
This method is often deployed to remove build up on surfaces such as essential bearings and bushings. Selective plating enables plating of localized areas with close tolerance and wall thickness. Unique advantages are also offered by some metals in applications such as adjoining parts in soldering and welding.
Metals that improve physical properties at joints can be brush plated to make soldering and welding easier. The process can also serve to increase the strength of the joints. Sometimes, the need is felt for enhanced conductivity only in certain parts of the component rather than the whole surface. Brush plating helps bring these properties to electrical components that rely on precious metals.
If the components have an odd shape or are too large in size, and heavy, they could be unsuitable for electrolyte baths. Brush plating can get these parts plated with speed and accuracy. Odd components that are used in critical applications can be made more flexible by changing their properties through brush plating.
Alloys and metals used in plating
A wide variety of metals are used in plating components. This is done using the electroplating, electroless plating, or selective plating processes. Often, the type of metal to be used depends on the type of application. Metals and alloys have specific advantages in plating. They can give a finished metallic look to improve aesthetics. They can also change a component’s chemical, physical, and mechanical properties for better performance under stress.
Here are some of the important metals and alloys used in plating:
Gold:
Gold plating is generally applied to small components due to the high price of the metal. Apart from jewellery, gold plating is applied to components such as connectors as gold has high electrical conductivity and resistance to oxidation. Gold remains a coveted metal due to its unique physical and mechanical properties. It is much rarer than copper, which also displays similar properties.
Silver:
Silver also possesses high electrical conductivity. It tends to be highly valued and is used mostly in jewellery and decorations. However, it can enhance the electrical conductivity of components. Even so, the benefits conferred by silver may not hold over the long term, and many other cheaper metals perform better than silver in a number of applications.
Copper:
Unlike gold or silver, is popular in industry for its lower cost and high electrical conductivity. As well as its alloys, it is used as plating material in various sectors. It is used widely in the manufacturing of circuit boards and electronic components. In addition to high corrosion resistance and heat transfer ability, copper also possesses a high plating efficiency. Even though stainless steel costs less than copper, its thermal conductivity is only 1/30 that of copper. This makes copper the metal of choice for heat transfer applications.
Zinc:
Zinc is used to plate a wide array of components from automotive parts, fasters, and nails to currency coins. Galvanizing process often uses zinc through electroplating.
Nickel:
Nickle is more commonly used in electroless plating. Many objects of everyday use such as kitchenware, plumbing fixtures, doorknobs etc. are plated with nickel. The popularity of many nickel alloys also makes the metal the most widely used in plating.
Chromium:
Chromium can help reduce friction and improve resistance to corrosion. Apart from this, it is also used for decoration due to its shiny clean surface finish. Many components in the automobile sector are electroplated with chromium for decorative purposes.
Cadmium:
Cadmium is used mostly to plate components that require enhanced paint adhesion. Apart from this, the metal also increases corrosion resistance and protects the components from wear and tear. Almost all conductive metals can be plated with chromium, making it a widely used metal in all sectors across the industry.